Download high-quality and well-structured Bpharm 6th-Semester notes in pdf format. The notes are in easy language and easy to remember covering all the topics of the syllabus.
Syllabus
Unit 4
Evaluation of Drugs WHO & ICH guidelines for the assessment of herbal drugs Stability testing of herbal drugs.
Patenting and Regulatory requirements of natural products:
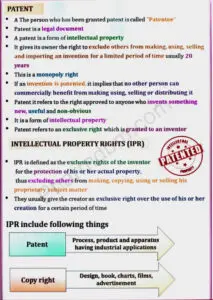
- Definition of the terms: Patent, IPR, Farmers right, Breeder’s right, Bioprospecting and Biopiracy
- Patenting aspects of Traditional Knowledge and Natural Products. Case study of Curcuma & Neem.
Regulatory Issues – Regulations in India (ASU DTAB, ASU DCC), Regulation of manufacture of ASU drugs – Schedule Z of Drugs & Cosmetics Act for ASU drugs.
Unit 4
You can also read this – 👇
Herbal Drug Technology 1 – Unit 1
Herbal Drug Technology 1 – Unit 2
Herbal Drug Technology 1 – Unit 3
Herbal Drug Technology 1 – Unit 5


