Free Download Pharmaceutical Jurisprudence Notes in pdf – Bpharm 5th Semester. High quality, well-structured and Standard Notes.
Welcome to Pharmdbm.com
Pharmdbm provides standard or well-structured Notes for Bpharm students. The notes are free to download. Each semester notes of Bpharm are available on www.pharmdbm.com.
In this post you can download notes of Pharmaceutical Jurisprudence (BP505T). All units are available to download for free.
Pharmaceutical Jurisprudence Notes Unit 1 – 5
UNIT – 1
Drugs and Cosmetics Act, 1940 and its rules 1945
UNIT – 2
Drugs and Cosmetics Act, 1940 and its rules 1945

UNIT – 3
Pharmacy Act –1948, Medicinal and Toilet Preparation Act –1955, Narcotic Drugs and Psychotropic substances Act-1985 and Rules
UNIT – 4
Study of Salient Features of Drugs and Magic Remedies Act and its
rules, Prevention of Cruelty to animals Act-1960, National Pharmaceutical Pricing Authority

UNIT – 5
Pharmaceutical Legislations, Code of Pharmaceutical ethics, Medical Termination of Pregnancy Act, Right to Information Act, Introduction to Intellectual Property Rights (IPR)

Syllabus of Pharmaceutical Jurisprudence
UNIT – 1
Drugs and Cosmetics Act, 1940 and its rules 1945:
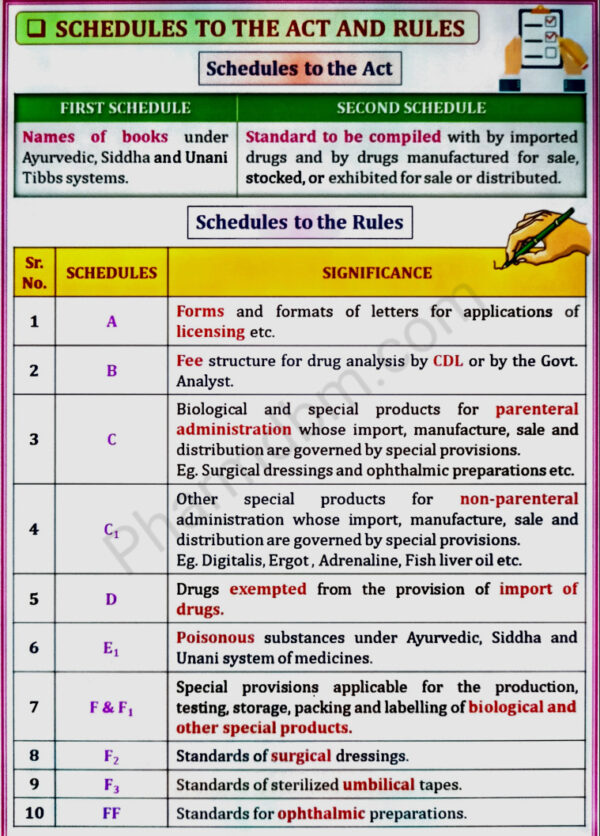
Objectives, Definitions, Legal definitions of schedules to the Act and
Rules
Import of drugs – Classes of drugs and cosmetics prohibited from import, Import under
license or permit. Offences and penalties.
Manufacture of drugs – Prohibition of manufacture and sale of certain drugs,
Conditions for grant of license and conditions of license for manufacture of drugs, Manufacture of drugs for test, examination and analysis, manufacture of new drug, loan
license and repacking license.
UNIT – 2
Drugs and Cosmetics Act, 1940 and its rules 1945.
Detailed study of Schedule G, H, M, N, P,T,U, V, X, Y, Part XII B, Sch F & DMR (OA)
Sale of Drugs – Wholesale, Retail sale and Restricted license. Offences and penalties
Labeling & Packing of drugs- General labeling requirements and specimen labels for
drugs and cosmetics, List of permitted colors. Offences and penalties.
Administration of the Act and Rules – Drugs Technical Advisory Board, Central drugs
Laboratory, Drugs Consultative Committee, Government drug analysts, Licensing
authorities, controlling authorities, Drugs Inspectors
UNIT – 3
Pharmacy Act –1948: Objectives, Definitions, Pharmacy Council of India; its
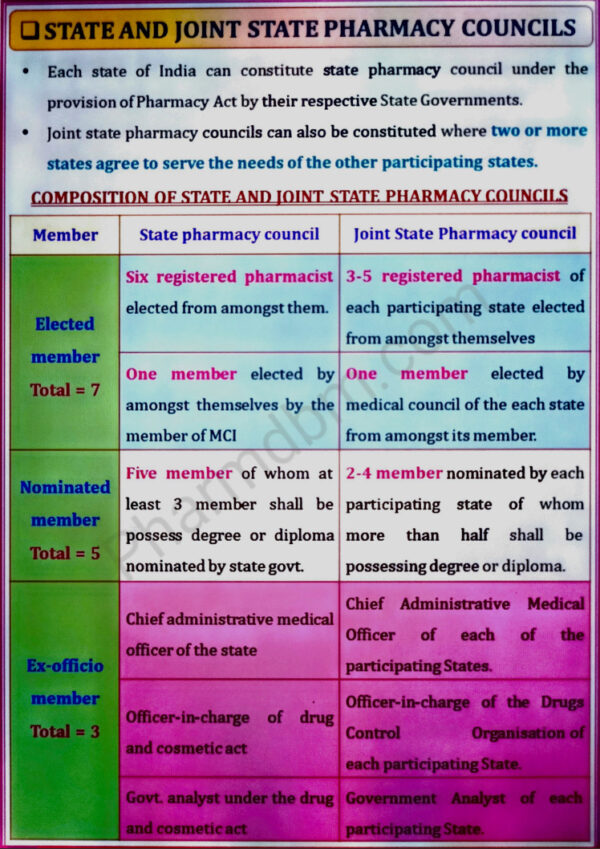
constitution and functions, Education Regulations, State and Joint state pharmacy
councils; constitution and functions, Registration of Pharmacists, Offences and penalties.
Medicinal and Toilet Preparation Act –1955: Objectives, Definitions, Licensing, Manufacture In bond and Outside bond, Export of alcoholic preparations,
Manufacture of Ayurvedic, Homeopathic, Patent & Proprietary Preparations.
Offences and Penalties.
Narcotic Drugs and Psychotropic substances Act-1985 and Rules: Objectives,
Definitions, Authorities and Officers, Constitution and Functions of narcotic &
Psychotropic Consultative Committee, National Fund for Controlling the Drug
Abuse, Prohibition, Control and Regulation, opium poppy cultivation and production
of poppy straw, manufacture, sale and export of opium, Offences and Penalties
UNIT – 4
Study of Salient Features of Drugs and Magic Remedies Act and its
rules: Objectives, Definitions, Prohibition of certain advertisements, Classes of
Exempted advertisements, Offences and Penalties
Prevention of Cruelty to animals Act-1960: Objectives, Definitions, Institutional
Animal Ethics Committee, CPCSEA guidelines for Breeding and Stocking of
Animals, Performance of Experiments, Transfer and acquisition of animals for
experiment, Records, Power to suspend or revoke registration, Offences and Penalties
National Pharmaceutical Pricing Authority: Drugs Price Control Order (DPCO)- 2013. Objectives, Definitions, Sale prices of bulk drugs, Retail price of formulations,
Retail price and ceiling price of scheduled formulations, National List of Essential
Medicines (NLEM)
UNIT – 5
Pharmaceutical Legislations – A brief review, Introduction, Study of drugs enquiry
committee, Health survey and development committee, Hathi committee and
Mudaliar committee
Code of Pharmaceutical ethics D efinition, Pharmacist in relation to his job, trade, medical profession and his profession, Pharmacist’s oath
Medical Termination of Pregnancy Act
Right to Information Act
Introduction to Intellectual Property Rights (IPR)
Scope of Pharmaceutical Jurisprudence
This course is designed to impart basic knowledge on important
legislations related to the profession of pharmacy in India.
Objectives of Pharmaceutical Jurisprudence
Upon completion of the course, the student shall be able to understand:
- The Pharmaceutical legislations and their implications in the development and
marketing of pharmaceuticals. - Various Indian pharmaceutical Acts and Laws
- The regulatory authorities and agencies governing the manufacture and sale of
pharmaceuticals - The code of ethics during the pharmaceutical practice


