
Easy to download High quality and well structure notes of Industrial Pharmacy 2 for Bpharm 7th semester students.
Welcome to Pharmdbm.com.
Pharmdbm provide high quality notes for Bpharm students. The notes are well structured and easy to remember.
In this post you will get Notes of Industrial Pharmacy 2 (BP702T) . Downloads link are given below, your download will started after clicking on the download button.
Industrial Pharmacy 2 Notes Unit 1 – 5
UNIT – 1
Pilot plant scale up techniques
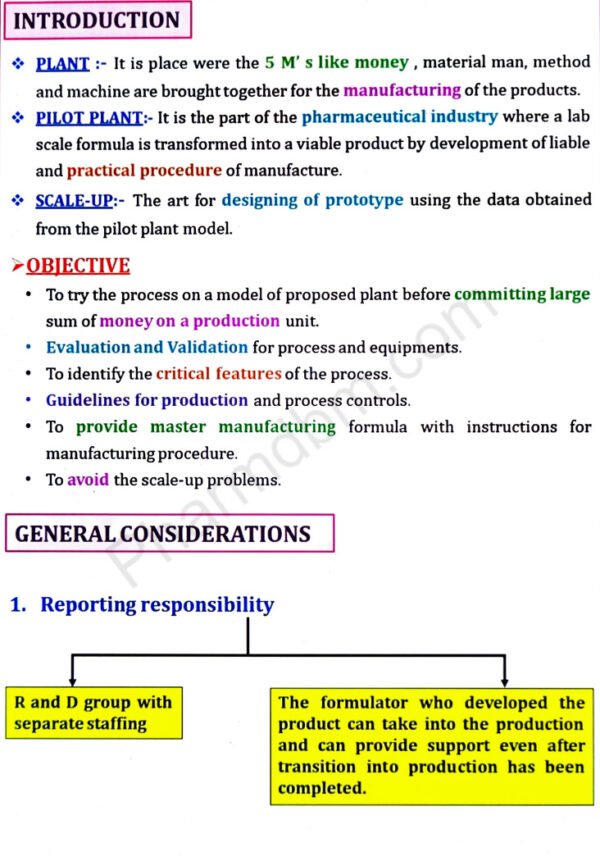
UNIT – 2
Technology Development & Transfer

UNIT – 3
Regulatory Affairs, Regulatory requirements for drug approval

UNIT – 4
Quality management system

UNIT – 5
Indian Regulatory Requirements

Scope of Industrial Pharmacy 2
This course is designed to impart fundamental knowledge on pharmaceutical
product development and translation from laboratory to market
Objectives of Industrial Pharmacy 2
Upon completion of the course, the student shall be able to:
- Know the process of pilot plant and scale up of pharmaceutical dosage forms
- Understand the process of technology transfer from lab scale to commercial batch
- Know different Laws and Acts that regulate pharmaceutical industry
- Understand the approval process and regulatory requirements for drug product
Syllabus of Industrial Pharmacy 2
UNIT – 1
Pilot plant scale up techniques: General considerations – including significance of
personnel requirements, space requirements, raw materials, Pilot plant scale up
considerations for solids, liquid orals, semi solids and relevant documentation, SUPAC
guidelines, Introduction to platform technology
UNIT – 2
Technology development and transfer: WHO guidelines for Technology Transfer(TT):
Terminology, Technology transfer protocol, Quality risk management, Transfer from R
& D to production (Process, packaging and cleaning), Granularity of TT Process (API, excipients, finished products, packaging materials) Documentation, Premises and
equipments, qualification and validation, quality control, analytical method transfer,
Approved regulatory bodies and agencies, Commercialization – practical aspects and
problems (case studies), TT agencies in India – APCTD, NRDC, TIFAC, BCIL, TBSE /
SIDBI; TT related documentation – confidentiality agreement, licensing, MoUs,
legal issues
UNIT – 3
Regulatory affairs: Introduction, Historical overview of Regulatory Affairs, Regulatory
authorities, Role of Regulatory affairs department, Responsibility of Regulatory Affairs
Professionals
Regulatory requirements for drug approval: Drug Development Teams, Non-Clinical
Drug Development, Pharmacology, Drug Metabolism and Toxicology, General
considerations of Investigational New Drug (IND) Application, Investigator’s Brochure
(IB) and New Drug Application (NDA), Clinical research / BE studies, Clinical Research
Protocols, Biostatistics in Pharmaceutical Product Development, Data Presentation for
FDA Submissions, Management of Clinical Studies.
UNIT – 4
Quality management systems: Quality management & Certifications: Concept of
Quality, Total Quality Management, Quality by Design (QbD), Six Sigma concept, Out
of Specifications (OOS), Change control, Introduction to ISO 9000 series of quality
systems standards, ISO 14000, NABL, GL
UNIT – 5
Indian Regulatory Requirements: Central Drug Standard Control Organization
(CDSCO) and State Licensing Authority: Organization, Responsibilities, Certificate of
Pharmaceutical Product (COPP), Regulatory requirements and approval procedures for
New Drugs


