
Free Download Industrial Pharmacy 1 Notes in pdf – Bpharm 5th Semester. High-quality, well-structured and Standard Notes that are easy to remember.
Welcome to Pharmdbm.com
Pharmdbm provides standard or well-structured Notes for Bpharm students. The notes are free to download. Each semester notes of Bpharm are available on www.pharmdbm.com.
In this post you can download notes of Industrial Pharmacy 1 (BP502T). All units are available to download for free.
Industrial Pharmacy 1 Notes Unit 1 – 5
UNIT – 1
Preformulation Studies
UNIT – 2
Tablets , Liquid Orals
UNIT – 3
Capsules (Hard gelatin & Soft gelatin), Pellets

UNIT – 4
Parenteral Products, Ophthalmic Preparations
UNIT – 5
Cosmetics, Pharmaceutical Aerosols, Packaging Materials Science

Syllabus of Industrial Pharmacy 1
UNIT – 1
Preformulation Studies: Introduction to preformulation, goals and objectives, study of
physicochemical characteristics of drug substances.
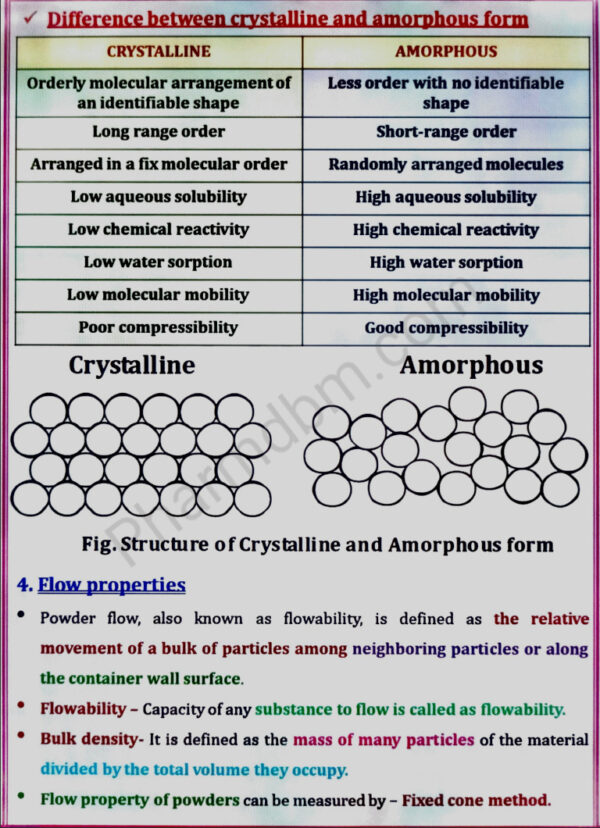
a. Physical properties: Physical form (crystal & amorphous), particle size, shape, flow
properties, solubility profile (pKa, pH, partition coefficient), polymorphism
b. Chemical Properties: Hydrolysis, oxidation, reduction, racemisation, polymerization
BCS classification of drugs & its significant
Application of preformulation considerations in the development of solid, liquid oral and
parenteral dosage forms and its impact on stability of dosage forms.
UNIT 2
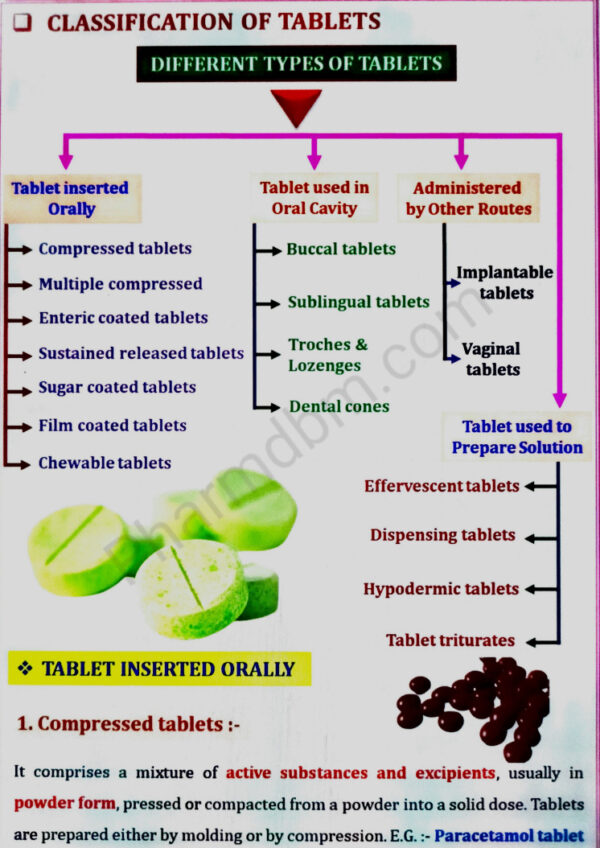
Tablets:
a. Introduction, ideal characteristics of tablets, classification of tablets. Excipients,
Formulation of tablets, granulation methods, compression and processing problems.
Equipments and tablet tooling.
b. Tablet coating: Types of coating, coating materials, formulation of coating
composition, methods of coating, equipment employed and defects in coating. c. Quality control tests: In process and finished product tests
Liquid orals: Formulation and manufacturing consideration of syrups and elixirs
suspensions and emulsions; Filling and packaging; evaluation of liquid orals
official in pharmacopoeia
UNIT – 3
Capsules:
a. Hard gelatin capsules: Introduction, Production of hard gelatin capsule shells. size
of capsules, Filling, finishing and special techniques of formulation of hard gelatin
capsules, manufacturing defects. In process and final product quality control tests
for capsules.
b. Soft gelatin capsules: Nature of shell and capsule content, size of
capsules,importance of base adsorption and minim/gram factors, production, in
process and final product quality control tests. Packing, storage and stability testing
of soft gelatin capsules and their applications.
Pellets: Introduction, formulation requirements, pelletization process, equipments for
manufacture of pellets
UNIT – 4
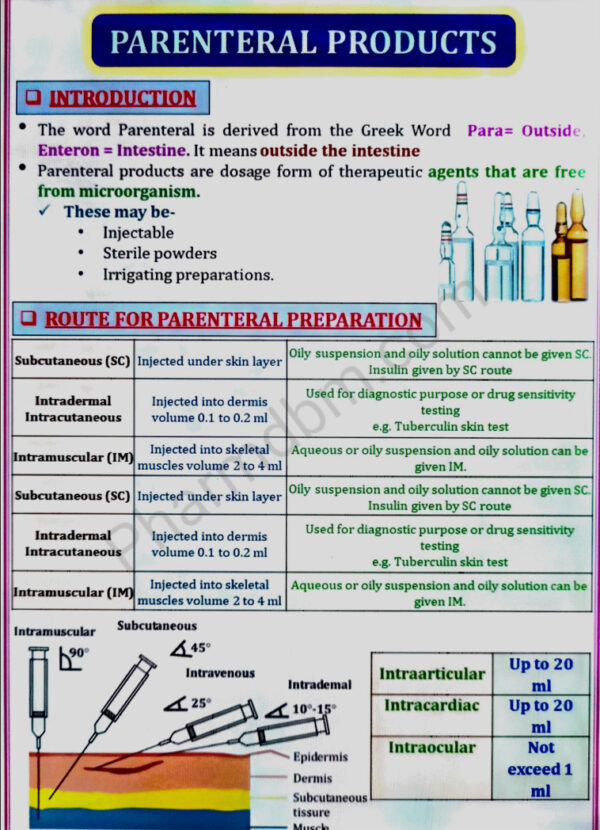
Parenteral Products:
a. Definition, types, advantages and limitations. Preformulation factors and essential
requirements, vehicles, additives, importance of isotonicity
b. Production procedure, production facilities and controls,
aseptic processing
c. Formulation of injections, sterile powders, large volume parenterals and
lyophilized products.
d. Containers and closures selection, filling and sealing of ampoules, vials and infusion
fluids. Quality control tests of parenteral products
UNIT – 5
Cosmetics: Formulation and preparation of the following cosmetic preparations:
lipsticks, shampoos, cold cream and vanishing cream, tooth pastes, hair dyes and
sunscreens.
Pharmaceutical Aerosols: Definition, propellants, containers, valves, types of aerosol
systems; formulation and manufacture of aerosols; Evaluation of aerosols; Quality
control and stability studies.
Packaging Materials Science: Materials used for packaging of pharmaceutical products,
factors influencing choice of containers, legal and official requirements for containers,
stability aspects of packaging materials, quality control tests.
Scope of Industrial Pharmacy 1
Course enables the student to understand and appreciate the influence of
pharmaceutical additives and various pharmaceutical dosage forms on the performance of
the drug product.
Objectives of Industrial Pharmacy 1
Upon completion of the course the student shall be able to:
- Know the various pharmaceutical dosage forms and their manufacturing
techniques. - Know various considerations in development of pharmaceutical dosage forms
- Formulate solid, liquid and semisolid dosage forms and evaluate them for their
quality


