
Free Download Pharmaceutical Quality Assurance Notes in pdf – Bpharm 6th Semester. High quality, well-structured and Standard Notes.
Welcome to Pharmdbm.com
Pharmdbm provides standard or well-structured notes for Bpharm students. The notes are free to download. Each semester’s notes for Bpharm are available on www.pharmdbm.com.
In this post, you can download notes on pharmaceutical quality assurance (BP601T). All units are available to download for free.
Pharmaceutical Quality Assurance Notes Units 1–5
UNIT 1
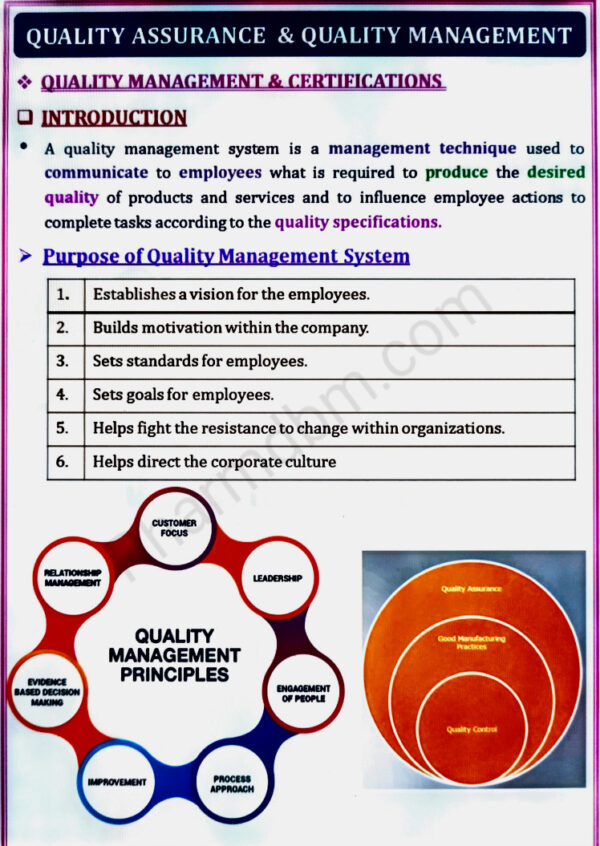
Quality Assurance and Quality Management concepts, Total Quality Management (TQM), ICH Guidelines, Quality by design (QbD), ISO 9000 and ISO14000, NABL accreditation
UNIT 2
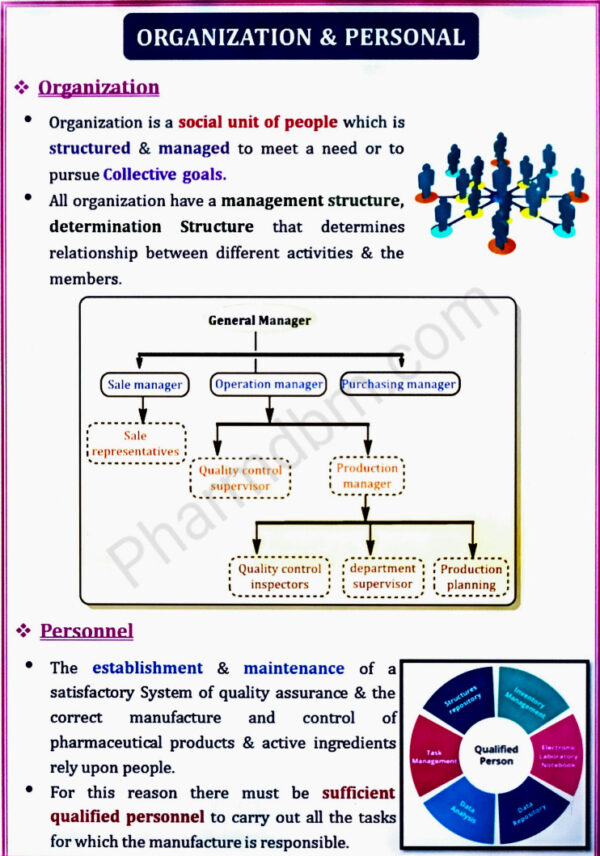
Organization and personnel, Premises, Equipments and raw materials,
UNIT 3
Quality Control and Good Laboratory Practices

UNIT 4
Complaints and document maintenance in pharmaceutical industry

UNIT 5
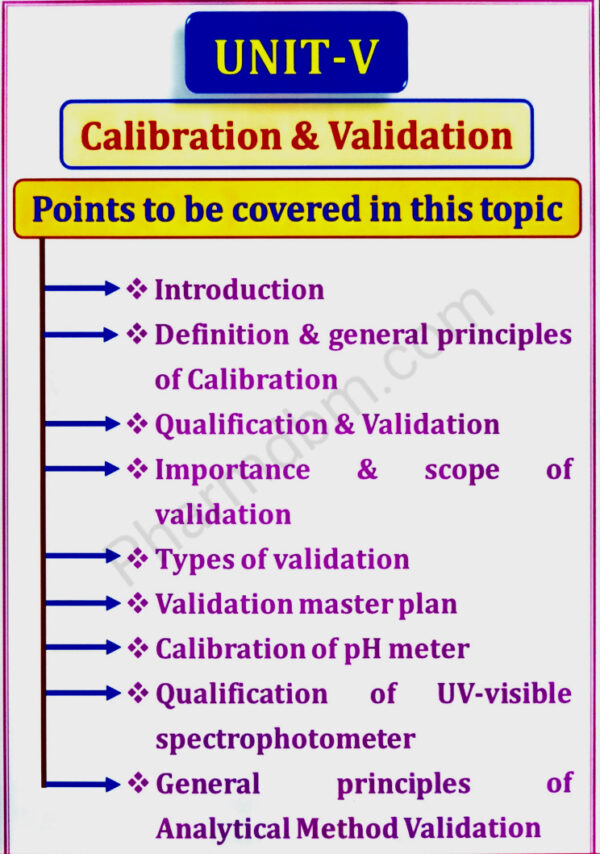
Calibration and Validation, Warehousing
Bpharm 6th Semester Notes:
- Medicinal Chemistry 3
- Pharmacology 3
- Herbal Drug Technology
- Biopharmaceutics and Pharmacokinetics
- Pharmaceutical Biotechnology
- Pharmaceutical Quality Assurance
Scope of Pharmaceutical Quality Assurance
This course deals with the various aspects of quality control and quality assurance in the pharmaceutical industry. It deals with important aspects like CGMP, QC tests, documentation, quality certifications, and regulatory affairs.
Objectives of Pharmaceutical Quality Assurance
Upon completion of the course, students shall be able to:
1. Understand the cGMP aspects of the pharmaceutical industry
2. Appreciate the importance of documentation.
3. Understand the scope of quality certifications applicable to pharmaceutical industries.


